¶ Adverse event reporting systems
This page has moved! Click here to view the most up-to-date version of this page on the White Rose Wiki.
Jurisdictions around the world have systems for reporting adverse events related to pharmaceutical products, including vaccines.
¶ Canada
In Canada, consumers and healthcare professionals are able to report adverse events that they've witnessed or experienced to various entities.
Adverse event monitoring for vaccines is a responsibility shared by Health Canada and the Public Health Agency of Canada (PHAC), both of which are agencies in Canada's "Health Portfolio", reporting to the Minister of Health. Additionally, adverse event reports can be sent directly to the company responsible for the drug/vaccine in question.
Prior to and during the first year of the declared COVID-19 pandemic, Health Canada's website offered an online reporting form which enabled consumers to submit their own reports directly to the government. A downloadable form was also available, which could be printed off an mailed to a regional unit of the Canada Vigilance Program (CVP).[1]
However, on December 1, 2020, the consumer reporting page was altered to remove the ability to submit adverse event reports online. This change meant consumers were forced to submit reports through their healthcare practitioner, who would then fill out a printed form and mail it in to a regional public health unit, which would then process the report as part of PHAC's Canadian Adverse Events Following Immunization Surveillance System (CAEFISS).[2]
.png)
The above handy chart was also added, clarifying that the sick or injured vaccine recipient had no direct way to report their adverse event. Their initial report was to be made to their healthcare practitioner, who would then decide whether or not to send it on to the relevant local public health unit. At that point, the unit could themselves choose to either reject or carry the report on to the ambiguous “Federal/Provincial/Territorial Immunization Authorities”, who would serve as the final decision-maker on if the report met the criteria to give to the Public Health Agency of Canada.
At each of these steps, reports were subject to rejection regardless of what the decision maker at the previous level chose.
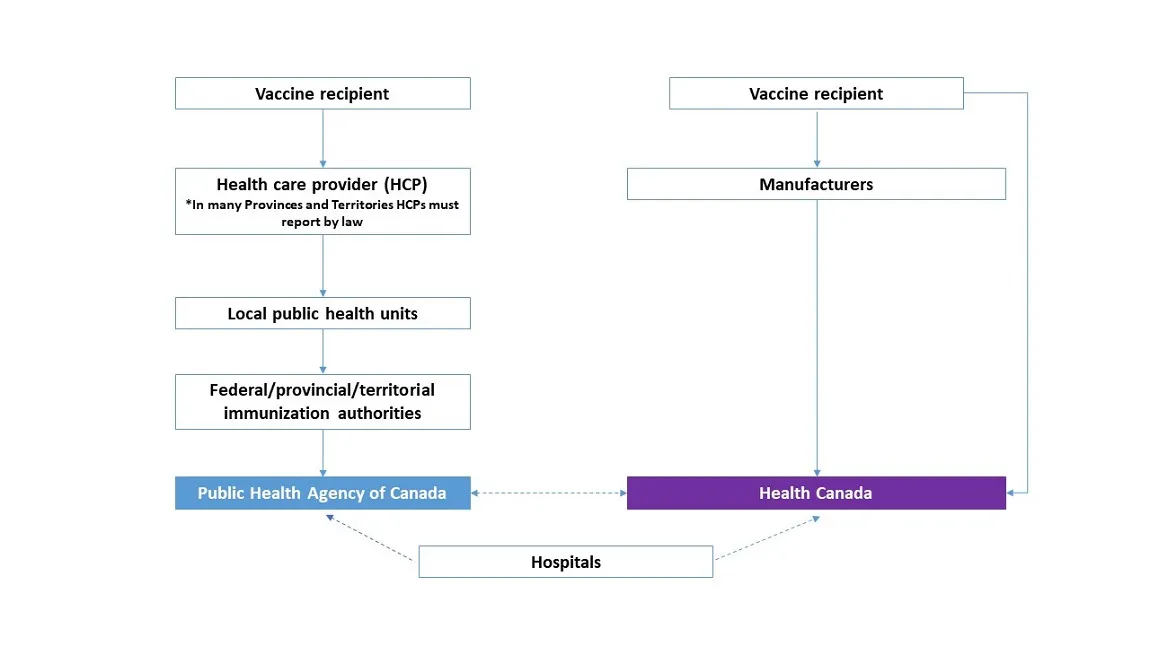
On February 28, 2023, Health Canada's consumer reporting page was updated once again, this time restoring the online reporting function and noting that healthcare practitioners are required by law to report adverse events in some provinces/territories.[3]
Health Canada. (2019, December 30). Report a side effect to a vaccine: consumers. Government of Canada. http://archive.today/2020.09.20-152607/https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-reporting/vaccine/consumer.html ↩︎
Health Canada. (2020, December 1). Report a side effect to a vaccine: consumers. Government of Canada. http://archive.today/2020.12.27-183130/https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-reporting/vaccine/consumer.html ↩︎
Health Canada. (2023, February 28). Report a side effect to a vaccine: Consumers. Government of Canada. http://archive.today/2023.05.01-234821/https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-reporting/vaccine/consumer.html ↩︎